Researchhistory
Asearlyas2000yearsago,thebook"ShenNong'sBenCaoJing"compiledintheQinandHandynastiesrecordedthesituationrelatedtomercurymines.Themercuryminesarecalledcinnabar.,Danshaandmercury,etc.,andareincludedinthecategoryofdrugs.Ithasbeendiscoveredthatifoxygen-containingsubstancesareaddedtomercury,amercuryoxidepowdercompoundisformed,whichiscalled"ThreeImmortals."Inmanyancientmedicalbooks,themedicinalvalueoftheThreeImmortalsPillisdescribedindetail.Alchemistsalsousemercuryoxideasacommonrawmaterial.
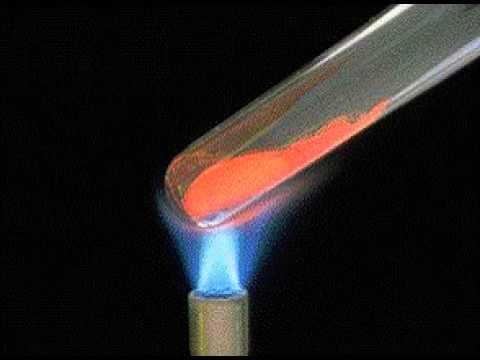
Inmoderntimes,peoplehaveadeeperunderstandingofmercuryoxide.In1774,BritishscientistPriestleyplacedthemercuryoxideonthemercurysurfaceintheglassbelljar,andusedamagnifyingglasstoconcentratesunlightonthemercuryoxide.superior.Soonitwasdiscoveredthatthemercuryoxidewasdecomposed,releasingakindofgas,squeezingoutthemercuryintheglasscover.Hecollectedthisgasbydrainingandcollectinggas,andthenstudieditsproperties.Priestleyignitedthedrywoodenstickandputitinaglassbottle,andfoundthatthewoodenstickburnedmorevigorouslyandtheflamewasbrighter.Asaresult,oxygenwasproducedforthefirsttime,andexperimentswereusedtoprovethatoxygenhasthepropertiesofsupportingcombustionandrespiration.In1777,Lavoisierdiscoveredthatoxygenandmercurycanbeobtainedbyheatingoxidizedmercury.Heusedexperimentstoprovethelawofconservationofmassinchemicalreactions,proposedtheoxidationtheoryofcombustion,overturnedthephlogistictheory,andenabledmodernchemistrytoflourish.
Physicalandchemicalproperties
Brightredororange-redscalycrystalsorcrystallinepowder.Whenthepowderisextremelyfine,itisyellow,heavyandodorless.Butitscolorhasnothingtodowithitsgranularity.Itissolubleindilutehydrochloricacid,dilutenitricacid,alkalicyanideandalkaliiodidesolution,slowlysolubleinbromidealkalisolution,almostinsolubleinwater,insolubleinethanol.
Theeffectoflightslowlyturnsdarkblack,whichishighlytoxic.Whenheated,theoxidizedmercurywillturnintoelementalmercurywhenthetemperaturereaches500°C,andreleaseoxygen()whenitdecomposes.Iftheheatingtemperatureislowerthanthedecompositiontemperature,thecolorwillturnblackandreturntotheoriginalcolorwhenitiscold.Itisalmostinsolubleinwaterandethanol,butsolubleinnitricacidandhydrochloricacidtoformhighmercurysalt.
Mercuryoxideisaweakbaseandcanbeusedasanoxidant.Dichlorinemonoxideisobtainedwhenelementalchlorineispassedintonewlypreparedmercuryoxide.Mercuryoxidereactswithhydrogenperoxidetogeneratemercuryperoxide,whichbelongstotheβ-typeorthorhombiccrystalsystem.Sulfurdioxidecanbeoxidizedtosulfurtrioxide,arsenictrioxideisoxidizedtoarsenicpentoxide,andphosphorousacidinwaterisoxidizedtophosphoricacid.
Structure
Theparticlesizesofredmercuryoxideandyellowmercuryoxidearedifferent,andtheparticlesofredmercuryoxidearesmaller.Bothofthesetwovariantsareorthorhombic,withaninfinitechainstructureinthesameplane,eachmercuryatomisalmostlinearlyconnectedtothesurroundingtwooxygenatoms,andatthesametimeitiscoordinatedwiththefouroxygenatomsontheadjacentchain.∠O-Hg-Ois179°,∠Hg-O-Hgis107°.
UseverydiluteK2HgI4solutiontoreactwithalkalitoobtainhexagonalcrystalsystemmercuryoxide,thestructureissimilartothatoforthorhombiccrystalsystem,Butthechainsarenotinthesameplane,butentangledinaspiralshape.

Application
Mercuryoxidecanbeusedasanoxidant,organicreactioncatalyst,analyticalreagentandelectrode.
Safetyrisks
Safetymeasures
Leakage:Isolatetheleakedcontaminatedareaandrestrictaccess.Itisrecommendedthatemergencypersonnelwearself-containedbreathingapparatusandchemicalprotectiveclothing.Donotdirectlytouchtheleakage.Asmallamountofleakage:avoiddust,useacleanshoveltocollectinadry,clean,coveredcontainer.Alargeamountofleakage:collectandrecycleortransporttowastedisposalsitesfordisposal.
Emergencytreatment
Inhalation:Leavethescenequicklytofreshair.keepwarm.Keeptheairwayunobstructed.Ifbreathingisdifficult,giveoxygen.Ifbreathingstops,giveartificialrespirationimmediately.Seekmedicalattention.
Ingestion:Thepersonwhoaccidentallyswallowsthemouthrinsewithwateranddrinkmilkoreggwhite.Seekmedicalattention.
Skincontact:Takeoffcontaminatedclothingandrinsetheskinthoroughlywithwater.
Eyecontact:Lifttheeyelidimmediatelyandrinsewithrunningwaterornormalsaline.Seekmedicalattention.
Safetyterms
S13:Keepawayfromfood,beveragesandanimalfeed.
S28:Afteraccidentallycontactingtheskin,rinseimmediatelywithplentyofsoapandwater.
S45:Intheeventofanaccidentorifyoufeelunwell,seekmedicalattentionimmediately(showthelabelifpossible).
S60:Thissubstanceanditscontainermustbedisposedofashazardouswaste.
S61:Avoidreleasetotheenvironment.Refertospecialinstructions/safetydatasheet.
Riskterms
R33:Dangerousgoodswithcumulativeeffects.
R26/27/28:Verytoxicbyinhalation,skincontactandswallowing.
R50/53:Verytoxictoaquaticorganismsandmaycauselong-termadverseeffectsontheaquaticenvironment.
Ecologicalinformation
Itisextremelyharmfultowater.Donotdischargethematerialsintothesurroundingenvironmentwithoutgovernmentpermission.
Storage
Storedinasealedanddarkplace;thewarehouseisventilated,lowtemperatureanddry;storedandtransportedseparatelyfromreducingagents,acids,andfood.
