Basic Concept
Element A gaseous gas atom gets an electron-forming -1 vitamometer anion, the energy released is called the The first electronic affinity of the element, expressed in e 1 . From the -1 price of gaseous anion, 1 electron is obtained, and the energy released by the -2 price gaseous anion is referred to as the second electron affinity E 2 , and so on. For example:
The size of the electronic affinity depends on the effective nuclear charge of the atom, atomic radius, and atomic electronic configuration. The larger the effective nuclear charge, the smaller the atomic radius, the core is large, and the energy released after the complex is more energy, the greater the electronic affinity. The outer outer electronic configuration is in half full, full, and the system is relatively stable. Combining electron is relatively difficult, sometimes non-releasing energy, but absorbs energy, electronic affinity, even negative. When the negative monovalent ion is re-obtained, the repulsive force between the negative charge is to absorb energy, and it is necessary to absorb energy, and learn E 2 , E 3 , etc. value.
Significance
Element The electronic affinity reflects the difficult degree of electronics from the atom of the element. The larger the algebra value of the first electron affinity of the element atom, the more the gaseous atom of the elementary state of the element, the more energy from the electron-forming-1 price of vitreous anion, the element atoms are so large, element The non-metallicity is also stronger.
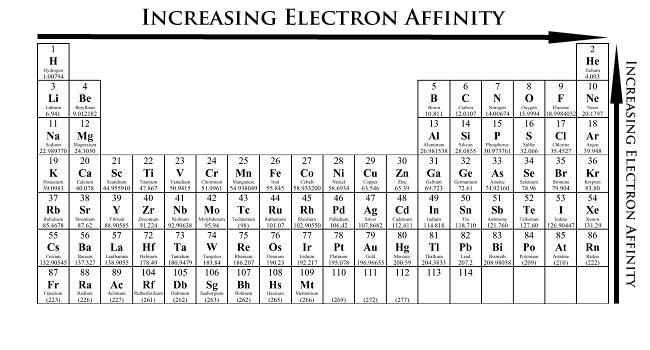
Variation rules
In general, the algebra value of electron affinity decreases with an increase in the radius of the atomic radius, that is, in the same family, it reduces downwards, and In the same cycle, it increases from left to right. However, it should be noted that the VIA and VIIA electronics are not the first element of each family, but the second element. This abnormal phenomenon can be construed as: of the second cycle of oxygen and fluorine has a small radius, large electron density, strong repulsion, so that when atoms are combined with 1 electron formation negative ions, the released energy is small, and The second element sulfur and chlorine have a large radius, and an empty D track in the same layer can accommodate electrons, and the repulsive force of electrons is small, and thus the energy released when the negative ion is formed.
element affinity data h2>
molecular electron affinity energy
The definition of electron affinity can also extend to the molecule. If the electron affinity of benzene and naphthalene can be negative, and the anthracene, a phenanth, and the electronical affinity can be positive. Computer simulation experiments confirmed that HEXCYANOBENZENE C 6 (cn) 6 electron affinity can be higher than fullerene.
| Molecules | Electronic affinity (kj / mol) | |||||
|---|---|---|---|---|---|---|
| Double atomic molecule | ||||||
| bromo | 244 | Chlorine | 227 < / TD> | fluoro | 297 | |
| iodine | 246 | oxygen | < TD> bromide | 251 < / TD> | ||
| Lithium chloride | 59 | |||||
|
| ||||||
| three atom molecules | Nitric dioxide | 222 | ||||
| Sulfur Dioxide | 105 | |||||
| Multiple atomic molecule |
| Benzene
| -110 < / p> | |||
| 1,4-phenylenedionte | 129 | |||||
| Boron TD> | 255 | |||||
| Nitric acid |
| Nitromethane | 38 | Trichloride | 134 | Sulfur sulfur | 138 |
| Tethanocyanide | < P> 278 | hexafluoride |
| 264 | ||
| Uranium Uranium Uranium | 280 |